Analyze and visualize bulk fluorescence tracing data#
Analysis and visualization of tracing data (e.g., anterograde or retrograde) in brains imaged post-hoc in a standard coordinate space.
Introduction#
The primary objective of tracing experiments in neuroscience is to elucidate the pathway specificity and establish connection relationships between neurons and various brain areas. By understanding these intricate connections, we can gain a deeper understanding of how the brain generates behavior. Therefore, it becomes imperative to quantify bulk-labeled axonal projections in images, as this process serves as a fundamental step in unraveling the intricate web of neuronal connectivity and its corresponding functionality.
Here we present a tool for the analysis of tracing axonal projections in post-hoc brain imaging data within a common coordinate space. This tool is integrated into brainreg, within the Brainglobe suite of computational neuroanatomy tools. Leveraging existing segmentation plugins available in napari, our tool imports and integrates these segmentation results into BrainGlobe, allowing for analysis of bulk axonal projections within the context of an anatomical atlas.
Before tracing bulk axonal tracks#
Tracing Compound Selection: Choose an appropriate tracing compound (or multiple ones if you plan to use different colors) based on the specific requirements of your experiment.
Injection: Carefully inject the tracing compound into the target region of the brain. The compound should be injected in a controlled manner to ensure accurate delivery and minimize damage to surrounding tissue.
Incubation: Allow sufficient time for the tracing compound to be taken up by neurons and transported along the axonal projections. The duration of the incubation period can vary depending on the specific compound and experimental requirements.
Tissue Processing: Sacrifice the animal and extract the brain for further processing. The animal is anaesthetised and perfused with 4% PFA following standard perfusion protocols. The brain is carefully extracted and left in 4% PFA overnight.
Note
To ensure high quality image registration, it is essential that the brain is properly perfused in order to decrease the autofluorescence of blood vessels. It is also important that the brain is extracted from the skull carefully in order to avoid tissue damage.
The brain is then thoroughly washed with 100mM PBS and imaged (e.g. by Serial Section 2-Photon Tomography). Image channels as needed according to number of tracing compounds (and extra channel is acquired as the background fluorescence only).
Brain registration to an atlas#
To track the bulk axonal projections into the standard space, the brain must first be registered to an atlas using brainreg.
Before registration, brainreg needs to be installed, please follow the instructions here. Once installed, we can proceed to register the imaged brain.
Note
Make sure you activate your conda environment before starting
You will need:
The path where the brain image stack (signal channels) is located
The path where the brain stack (background fluorescence channel) is located
The path where you want the registration result to be saved
The resolution at which the brain was imaged
To register your brain to an atlas, please follow the instructions for brainreg (using the napari plugin) here.
A new output directory has been created, which contains the registered brain. We are now ready to use external plugins to find a mask that covers the axonal projections.
Bulk axonal track detection#
Before we can proceed to register the axonal tracks in the imaged brain we need to install additional Napari plugins.
In this tutorial we will use the
napari-simpleitk-image-processing plugin. This
plugin can either be installed via the napari plugin menu
or directly from PyPI on the command-line:
pip install napari-simpleitk-image-processing
Note
Alternative plugins can be used to generate the segmentation mask for the area of interest. In this case, we will use
the Otsu[1] thresholding method from napari-simpleitk-image-processing an example.
To use Otsu’s method from the napari-simpleitk-image-processing plugin, follow these steps:
Open napari
Choose the Otsu thresholding option from the plugin menu:
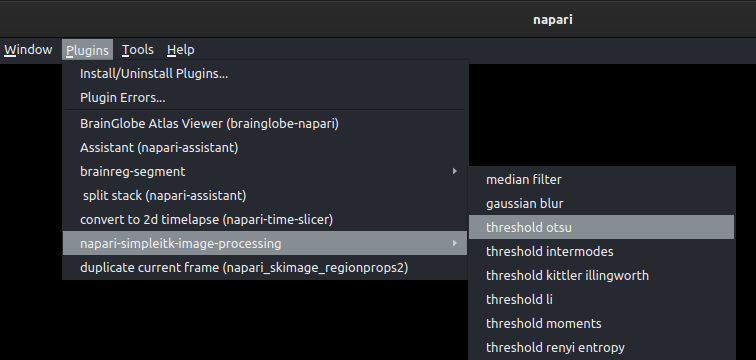
Select the image layer to be thresholded and click
run. This will automatically segment your image using Otsu’s algorithm. This will create a new labels layer.Follow the instructions for 2/3D region analysis to analyse the distribution of the thresholded voxels.
Instructions by Sara Mederos.